Oocyte/ Embryo/ Sperm donation
Oocyte/ Embryo/ Sperm donation
Egg donation requires in vitro fertilization (IVF), as the eggs are removed from one woman, fertilized in the laboratory, and the resulting embryo is transferred to the recipient’s uterus. Sperm from either the recipient’s male partner or a sperm donor are used to fertilize these eggs in the laboratory.
EGG DONATION
The first pregnancy resulting from egg donation was reported in 1984. Since then, egg donation has helped many struggling with infertility to conceive. With egg donation, the intended parents will have a genetic link to the child only if they contribute the sperm used to fertilize the egg. Egg donation requires in vitro fertilization (IVF), as the eggs are removed from one woman, fertilized in the laboratory, and the resulting embryo is transferred to the recipient’s uterus. The basic steps of egg donation with IVF are described below. For more information about IVF, please see the ASRM patient education booklet titled, Assisted Reproductive Technology.
- The first step is to find an egg donor. This can be either someone known to the intended parent(s) or an anonymous donor.
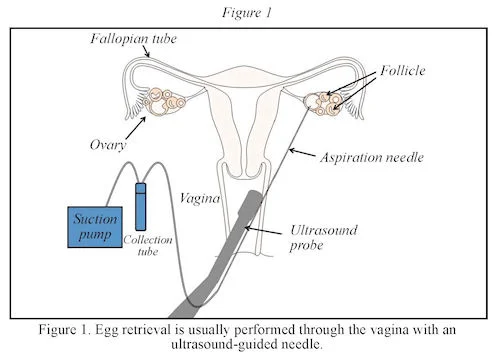
- The donor takes medication to stimulate her ovaries to produce multiple eggs and the eggs are collected. Sometimes, to share costs, the eggs from an egg-donation cycle are split among several recipients.
- Sperm from either the recipient’s male partner or a sperm donor are used to fertilize these eggs in the laboratory.
- An embryo (fertilized egg) is chosen and transferred to the uterus (womb) of the intended carrier and, hopefully, a pregnancy is established. The intended carrier can be the intended parent or another woman (gestational carrier), depending on the circumstances.
Who are Egg Donors?
There are several ways of obtaining donor eggs:
Anonymous donors: Women who are not known to the recipient(s). Donors may be found through egg donation programs or through agencies.
Known (directed donors): Women who are known to the recipient(s). The donor is generally a close relative or friend. In some instances, recipients advertise directly for donors in newspapers or on the internet. In these circumstances, the recipient(s) and the donor are known to each other in a limited way, and meet without an intermediary program or agency. Recipients should be cautious if recruiting donors directly without having an intermediary program or agency screen donors or without seeking legal counsel.
IVF programs: Women undergoing IVF may agree to donate their excess eggs to infertile patients. This source of donors is limited because this type of donation can be seen as coercive, particularly if the donors are offered a financial discount on their own IVF cycle.
Preparation of the Recipient for Embryo Transfer
In cycles where the embryos are transferred without being frozen (fresh cycles), the donor’s and recipient’s cycles must be synchronized so that the recipient’s uterine lining (endometrium) is ready for the embryo when it is transferred. For cycles where the embryo is frozen, the menstrual cycles of the recipient and donor do not need to be synchronized, but the recipient’s endometrium must still be prepared, using medication, to receive the embryo before the embryo is transfererd to her.
There are many ways to do this, but the principle of hormonal preparation is similar among individual protocols. Women whose ovaries are functioning are given a GnRH-a to temporarily suppress their menstrual cycle. When the donor starts medications to stimulate her ovaries, the recipient is given estradiol to stimulate the endometrium to develop. Estradiol may be given in the form of an oral pill, transdermal patch, or injection. Ultrasound and blood tests may used to assess the readiness of the endomtritum during this time. The recipient typically begins progesterone on the day after the donor receives the ovulation trigger medication. Progesterone causes specific changes within the endometrium that enable the embryo to implant. Progesterone may be given by intramuscular injection, vaginal gel, or tablet.
Embryos are transferred into the recipient’s uterus, usually within three to five days after the eggs are fertilized in the laboratory. The embryo transfer (Figure 2) is performed by passing a small catheter with the embryo(s) through the cervix and into the uterus. If the recipient couple has extra embryos, these embryos may be cryopreserved (frozen) and used later in additional attempts to achieve a pregnancy.
Pregnancy Rates with Egg Donation
The pregnancy rate with egg donation depends on many factors but generally not on the age of the recipient. Success rates compiled by the Society for Assisted Reproductive Technology (SART) for the year 2015 show that the average live-birth rate per egg-donation cycle was 46.2% overall (50.4% for fresh cycles and 38.4% for frozen cycles) across all eggdonor programs. The major risk for egg donation is multiple gestations. In 2015, of the 9,197 cycles resulting in an embryo transfer, 4,249 resulted in a live birth. Of these live births, 74% resulted in singleton live births and 25.5% resulted in twin live births. Because many of the pregnancies miscarry before the number of fetuses can be counted, the percentage of multiple pregnancies actually may be higher. The current recommendation to reduce the risk of multiple gestations is to limit the number of embryos transferred. Most programs will limit the number of embryos transferred to one if the donor is between the ages of 21 and 37. Transfer of a single high-quality embryo, called elective single-embryo transfer (eSET), helps minimize the risk of multiple gestation.
SPERM DONATION
Insemination using donor sperm has been practiced for over a century, although the first published reports of such were in 1945. Since the late 1980s, with the emergence of HIV, donor insemination (DI) has been performed only with frozen and quarantined sperm to allow for time to test the donors. FDA and ASRM guidelines recommend that sperm be quarantined for at least six months before being used.
Reasons for Sperm Donation
Currently, DI is appropriate when the male partner has severe abnormalities in his semen and/or reproductive system, which may be present at birth (congenital) or develop later (acquired) and in other situations. For instance:
- Azoospermia (absence of sperm) can be due to a blockage (obstructive azoospermia), such as congenital bilateral absence of the vas deferens (CBAVD) or previous vasectomy. Alternatively, azoospermia can be due to testicular failure (nonobstructive azoospermia) resulting from exposure to toxins like pesticides, radiation treatment, or chemotherapy.
- Severe oligozoospermia (decreased sperm count) or other significant sperm or seminal fluid abnormalities also are indications for DI.
- Ejaculatory dysfunction, such as inability to achieve or maintain an erection or to ejaculate, is a scenario where DI can be helpful.
- DI in place of an affected male’s sperm can help bypass significant genetic defects that can be passed to children.
- When there is no male partner, such as with single women who wish to become parents or lesbian couples who desire a pregnancy, but who lack a male partner, DI is needed for pregnancy.
Selection of Sperm Donors
Sperm donors should be of legal age and ideally less than 40 years of age to minimize the potential increased risks of older male parents. Like egg donors, sperm donors can be anonymous or known (directed). ASRM believes it is important that both anonymous and known donors undergo the same initial and periodic screening and testing process, whether or not they are intimate sexual partners of the recipient. The FDA requires that anonymous and directed sperm donors be screened for risk factors for, and clinical evidence of, communicable disease agents or diseases.
A donor is ineligible if either screening or testing shows the presence of a communicable disease or a risk factor for a communicable disease. A comprehensive medical questionnaire to evaluate the health of a donor and review of his family medical history is the primary focus in selecting a donor. Particular attention is paid to the potential donor’s personal and sexual history to exclude those males who are at high risk for communicable diseases including HIV, hepatitis, and other sexually transmitted diseases. A family medical health history is obtained for at least two generations of family members. Prospective donors must have a physical examination with screening for visible physical abnormalities, as well as testing for sexually transmitted diseases. Routine blood analysis includes documentation of the donor’s blood type. Current FDA regulations require infectious disease testing to be performed within seven days of all sperm donations. The sperm are collected by masturbation, concentrated into small volumes of motile sperm, and frozen or cryopreserved until used. For donors, testing for syphilis, chlamydia, gonorrhea, HIV-1, HIV2, human T-lymphotropic virus (HTLV)-I and HTLV-II, CMV, hepatitis B surface antigen, and hepatitis C antibody are performed prior to donation and thereafter should occur at six-month intervals, per FDA guidelines. Although the FDA exempts directed sperm donors from the six-month retesting requirement, ASRM recommends that directed donors be retested just as anonymous donors are retested. Comprehensive genetic testing may be impractical; however, at this time, ethnically based genetic testing is standard in most sperm banks.
It is recommended that all sperm donors, anonymous and directed, have a psychological evaluation and counseling by an MHP. The assessment should seek any psychological risks and evaluate for financial and emotional coercion. The donor should discuss his feelings regarding disclosure of his identity and plans for future contact. Psychological testing may be performed, if warranted.
The sperm donor should undergo a semen analysis, and the test sample should be frozen and thawed for evaluation. Sperm susceptibility to damage with freezing varies among individuals, as well as among samples of a given donor. Donors are considered if the post-thaw semen specimen meets minimum standards. In general, specimens should contain a minimum of 20 to 30 million motile (moving) sperm per milliliter after thawing. Post-thaw motility is generally in the range of 25% to 40%.
Two types of samples are offered by most sperm banks. Intracervical insemination (ICI) specimens are prepared for intracervical inseminations only. Samples must be washed if used for intrauterine inseminations (IUIs). Although sperm preparations for ICI are available, most reproductive endocrinology practices perform IUI. Both ICI and IUI semen samples are frozen and quarantined for a minimum of 180 days. They are not released until the donor is retested for communicable diseases and the results are negative.
In addition to medical information obtained from the donor, donors are asked to provide detailed information about their personal habits, education, hobbies and interests. Sperm banks may provide pictures of the donor and video or audiotapes from the donor. Donors may identify themselves as open to contact from any child conceived through DI once a child reaches legal age.
